
Here we study some clusters of aluminium doped with lithium and magnesium. We show that based on the jellium model shell filling, metal clusters can behave like ``super-atoms''. We show that in some situations this kind of ``super-atom'' behavior leads to such a cluster binding ionically with another metal atom. This is an interesting phenomenon, and can be used to investigate the extent to which the jellium picture is valid for a particular kind of clusters. It may also open up possibilities of designing clusters of desired stability, by choosing the kind of bonding one wants to have.
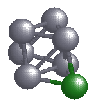 We first look at the cluster Al6Mg, where the six aluminium
atoms for an octahedron, and the Mg atom caps a triangular face, as
shown in the picture. Each Al atom has 3 valence electrons, which
makes the total number of electrons in the Al6 cluster 18.
One would recall that 18 is just two short of shell closing. A Mg atom
has two valence electrons, and will be happy to part with them. So, if
one forms a cluster Al6Mg, and the jellium picture works,
one should see a transfer of the two electrons from Mg to the
Al6 cluster.
We first look at the cluster Al6Mg, where the six aluminium
atoms for an octahedron, and the Mg atom caps a triangular face, as
shown in the picture. Each Al atom has 3 valence electrons, which
makes the total number of electrons in the Al6 cluster 18.
One would recall that 18 is just two short of shell closing. A Mg atom
has two valence electrons, and will be happy to part with them. So, if
one forms a cluster Al6Mg, and the jellium picture works,
one should see a transfer of the two electrons from Mg to the
Al6 cluster.We did a Car-Parrinello molecular dynamics calculation for the Al6Mg cluster and found out the lowest energy electronic state. We calculated the distribution of electrons in the cluster,
 |
|
You might think that this phenomenon is specific to Al and Mg. To
convince you that this is not so, we take another example. We take
 a Al6 clusters and two Li atoms. Lithium (Li) atom has one
valence electron which it can happily part with. So, with two Li atoms
and the Al6 cluster we should be able to observe the same
effect as in Al6Mg.
a Al6 clusters and two Li atoms. Lithium (Li) atom has one
valence electron which it can happily part with. So, with two Li atoms
and the Al6 cluster we should be able to observe the same
effect as in Al6Mg.
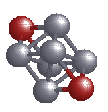
Again we take the octahedral Al6 and cap two Li
atoms on opposite sites, as shown in the figure. So, how does the  constant
density surface look like in this case? In the figure alongside, a
dimple at the lower right side in the constant density surface denotes
the location of an Li atom. This indicates that the electron has been
almost completely transferred to the Al6 cluster. So, the
Jellium picture actually works.
constant
density surface look like in this case? In the figure alongside, a
dimple at the lower right side in the constant density surface denotes
the location of an Li atom. This indicates that the electron has been
almost completely transferred to the Al6 cluster. So, the
Jellium picture actually works.
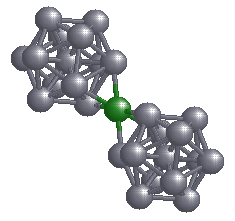
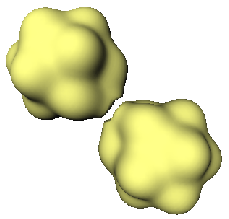
A cluster of Aluminium behaves like a "super-atom" and follows electronic shell filling rules like atoms. Here, the surprising fact is that although Al, Mg and Li are metals, the bonding between the cluster and an atom is ionic. Because of the fact that electron transfer takes place either from the cluster to the atom or vice-versa there is a polarization of charge between the cluster and the atom. Such clusters where electron transfer takes place, may behave like an ionic molecule, and hence it may be possible to form an ionically bonded solid out of such entities. The question whether this polarization will survive at all, if one tries to make a solid out of such entities, remains open. However, With this picture of a cluster ionically bonded to an atom, one can go ahead to speculate whether such ionic bonding is possible between two clusters, thereby leading to a formation of a ``cluster-molecule''. This is an interesting possibility, and will have bearing on what people are trying now, i.e., to make cluster assembled materials (see R. Palmer, New Scientist, 22 Feb. 1997).

|
|
 |
| 
|
In future one can investigate various kinds of clusters to find out where such a senario leads to a really stable cluster-molecule. It would be interesting to see how the chemistry of a cluster changes depending on how it is bonded to another cluster or an atom. It appears, one can now talk about electronegativity and electron affinity of a cluster.