
|
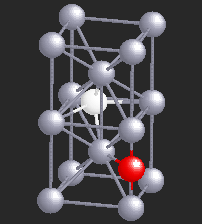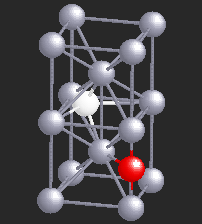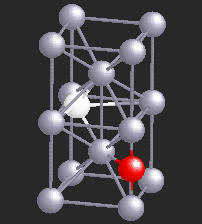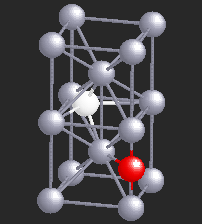
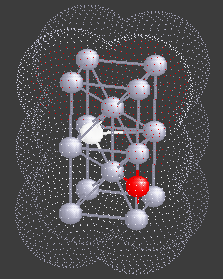
Hydrogen (H), being a small atom, if introduced in a Nb sample, can sit in the interstitial sites between the Nb atoms. At very low temperatures H is seen to move freely through the Nb lattice of atoms. However, if there are certain impurity atoms like Oxygen (O) or Nitrogen (N), present in the Nb sample, they form trapping centers for the otherwise freely moving hydrogen atoms. A typical location of an Oxygen atom in the Nb lattic is shown by in red in the figure.
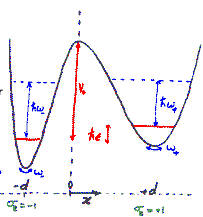
In the presence of Oxygen impurities, the H atom likes to stay around such impurity sites. It tries to sit at the site where its energy will be the lowest. It turns out that the two sites shown in the figure are the lowest energy sites for the H atom (shown in white). These sites are physically quite close, and also energetically identical for the H atom. The hydrogen atom near such sites "sees" a potential which is like a double-well. However, in a given sample of Nb, there are many such double-wells because there are many H atoms trapped around Oxygen impurities. The presence of other impurities affect the symmetry of the double-well, and it will in general be asymmetric, as shown in the figure below. It can stay in either of the two wells, but not in between. At high temperatures H atom has enough energy to jump the barrier between the two wells. At low temperatures it can stay in either of the two wells, but cannot jump from one to the other.
 This is true for things which follow Newton's laws. But a hydrogen atom
is too small to be contrained by Newton's laws. It takes advantage of
the laws of the Qunatum theory, which is a more fundamental theory of
nature, and is seen only when one goes to atomic scales. Even if the H
atom doesn't have enough energy to jump over the barrier, it can grow
fuzzy and just "tunnel" to the other site! Quantum mechanic
allows this. Left to itself at a particular site, the H atom will
execute a coherent clock like motion between the two sites. This is
called "quantum tunneling".
This is true for things which follow Newton's laws. But a hydrogen atom
is too small to be contrained by Newton's laws. It takes advantage of
the laws of the Qunatum theory, which is a more fundamental theory of
nature, and is seen only when one goes to atomic scales. Even if the H
atom doesn't have enough energy to jump over the barrier, it can grow
fuzzy and just "tunnel" to the other site! Quantum mechanic
allows this. Left to itself at a particular site, the H atom will
execute a coherent clock like motion between the two sites. This is
called "quantum tunneling".
There are basically three kinds of scattering of neutrons:
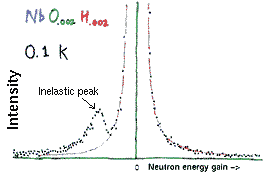
The energy gain/loss measured by Wipf and collaborators, at a temperature of 0.1K, is shown in the figure on the right. There is big peak at the center, which represents elastic scattering from immobile Nb atoms.
|
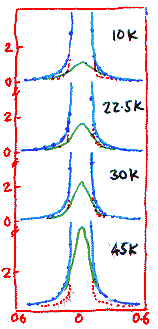
This, however, is just one part of the story. When the experiments were repeated at higher temperatures, inelastic scattering disappeared completely, and was replaced by a quasi-elastic peak. This, one would recall, indicates that the scattering atom is not moving coherently but incoherently. This means that the tunneling motion has been destroyed by something which is causing randomness.
The width of the quasi-elastic peak is supposed to quantify the rate at which the scattering atom is jumping randomly between two sites. Normally one expects that as the heat is turned on, the scattering atom is kicked around more and more and so the width of the quasi-elastic peak will increase with temperature. But here it was observed that the quasi-elastic peak actually narrows with the increasing temperature! This is really strange, for it means that as the temperature is increased, the atom tends to sit more on one site rather than jump around!
This is really weird - so let us try to see how this can happen. Recall that Nb is a metal. So the H atom which moves between the two sites, not only sees the double-well potential but also feels the effect of the sea of electrons formed by the outer electrons of Nb (see the picture alongside).
|
If one grinds through the calculation (S. Dattagupta and T. Qureshi, Physica B, 174 (1991) 262), one gets the following picture: